Abstract
Background
Myelodysplastic syndromes (MDS) are a collection of hematopoietic disorders with widely variable prognoses and treatment options. Pathologic diagnosis can be challenging and misdiagnosis can impact patient therapy and outcome. How commonly misdiagnosis occurs, and the severity of diagnostic errors, is not known. Here, we report interim analyses of patients (pts) with cytopenia and suspected MDS from the NHLBI National MDS Natural History Study (https://thenationalmdsstudy.net ClinicalTrials.gov: NCT02775383) assessing MDS occurrence and rates of agreement on classification of MDS/MDS-related disorders by local and centralized review.
Methods
Pts with cytopenias and clinically suspected MDS were identified between 6/16 and 6/18 from 84 participating centers in this ongoing multi-Institutional Cooperative Group study, with a goal of recruiting 2000 MDS (WHO 2016 subcategories), MDS/MPN or low blast count acute myeloid leukemia (AML, <30% blasts without core binding factor) and 500 cases with idiopathic cytopenia of undetermined significance (ICUS) from both NCI community oncology research program (NCORP) and lead academic participating sites. Centrally submitted clinical and pathologic data and bone marrow samples were analyzed by pathologists in the central laboratory & biorepository (CL/B) blinded to the original site's diagnosis, with a third-level review for cases with disagreement between the local and CL/B assignment. Disagreements in the 5 categories detailed in Figure 1 were considered clinically meaningful. Cases were assigned to longitudinal (MDS, MDS/MPN, ICUS, low blast count AML) versus cross-sectional (other cytopenias or cancers) cohorts after central classification based on clinical, pathologic, and cytogenetic features. Interrater-agreement was evaluated with the Kappa statistic.
Results
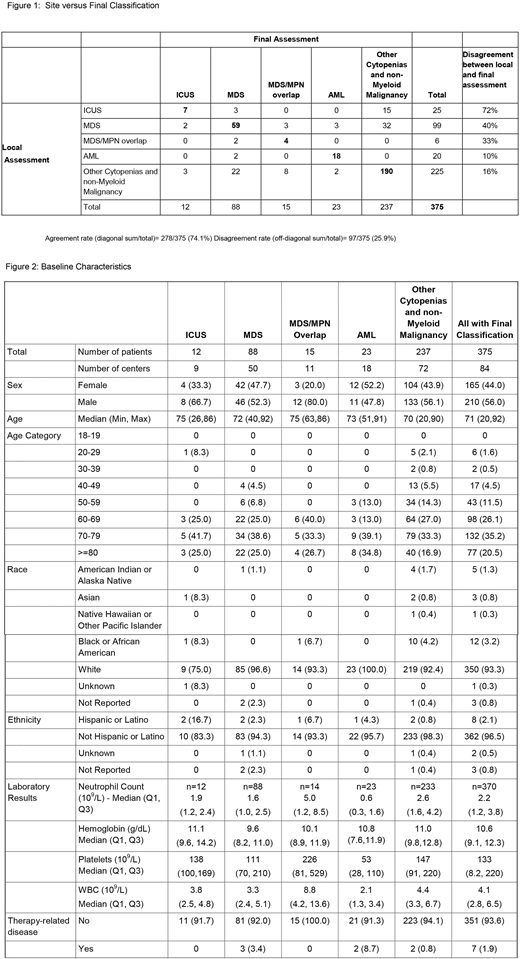
Of 375 pts for whom data and samples were submitted with completed classification, 88 (23%) had MDS, 15 (4%) MDS/MPN, 12 (3%) ICUS, 23 (8%) AML, and 237 (63%) other cytopenias (Figure 1). The median age of all pts was 71 years (range, 20-92), 44% were female, and median baseline blood counts and other baseline measures are in Figure 2. MDS pts had single lineage dysplasia (SLD, 0), SLD with ring sideroblasts (RS, 9 (10%)), multi-lineage dysplasia (MLD, 17 (19%)), MLD -RS (18 (20%)), excess blasts I (EB, 14 (16%)), EBII (19 (22%)), del(5q) (6 (7%)), and MDS-U (5(6%)). IPSS-R categories were defined in 51 of 88 MDS cases (58%): Very Low (27%), Low (43%), Intermediate (27%), High (16%), and Very High (14%).
Overall site/central agreement on diagnosis occurred in 225 cases (60%) with inconsistency associated with recognized site coding errors resolved in 54 cases (14%) without 3rd party review. Seventy-eight others (21%) were referred to 3rd level review; confirmation of CL/B classification occurred in 49/78 cases (63%), agreement with site in 13/78 (17%), and a different diagnosis in 16/78 (21%). Clinically meaningful changes in diagnoses between local and central review occurred 26% of the time (Figure 1, n=97/375 kappa =.53 95% CI (.45, .61)). Site assigned MDS was changed to ICUS or other cytopenia in 35% (n=34/99); and to AML in 3% (n=3/99). For cases with site assignment to other causes of cytopenias (225 of 375 cases, 60%), central assignment identified ICUS in 3, MDS/MPN overlap in 8, AML in 2 and MDS in 22, totaling a change in diagnosis in 16%. Of note, 60% (15/25) of ICUS diagnosed locally were interpreted as reactive marrow or normal according to central review.
Within MDS cases diagnosed locally, the greatest discrepancy was observed in the MDS-U classification, reported 31 times (31/99 31%) but confirmed in only 2 cases (6%), with 22 (71%) found to not have MDS. Across the study when compared to local assignment, central review changed the follow-up cohort assignment for 87 pts (23%).
Conclusions
In this well-characterized series of pts evaluated for MDS with bone marrow biopsy and paired site/central morphologic assessment, 40% of site diagnoses were changed at central review and site coding errors were common. In 26%, the changes were clinically meaningful, potentially affecting patient treatment and prognosis. In particular, site designation of MDS-U was an unreliable classification category, which could only partially be attributed to miscoding errors at the local site. Incorporating genomics data might help refine MDS diagnoses.
Bejar:Genoptix: Consultancy; Takeda: Research Funding; Celgene: Consultancy, Honoraria; Modus Outcomes: Consultancy; Astex/Otsuka: Consultancy, Honoraria; AbbVie/Genentech: Consultancy, Honoraria; Foundation Medicine: Consultancy. Komrokji:Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau. Scott:Agios: Consultancy; Novartis: Research Funding; Celgene: Consultancy, Research Funding; Alexion: Consultancy. Gore:Celgene: Consultancy, Research Funding. Sekeres:Opsona: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Opsona: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.